
Specialists of the World Health Organization keep track of progressive medical technologies, providing access to them to people around the world on their resource.
The WHO website containing the information that health care professionals from different countries can use to preserve people’s health has published the results of clinical trials of a new method of Russian scientists in the treatment of COVID-19, which underlies the operation of the TOR electromagnetic non-invasive therapy device.
The therapeutic potency of the device and the safety of using the method of pulsed electromagnetic radiation to combat the pandemic were confirmed during the execution of special instructions of the President of the Russian Federation V.V. Putin in 2020-2021.
As a result of clinical trials, the medical purpose of the TOR device was established as a means to accelerate the elimination (death) of SARS-CoV-2.
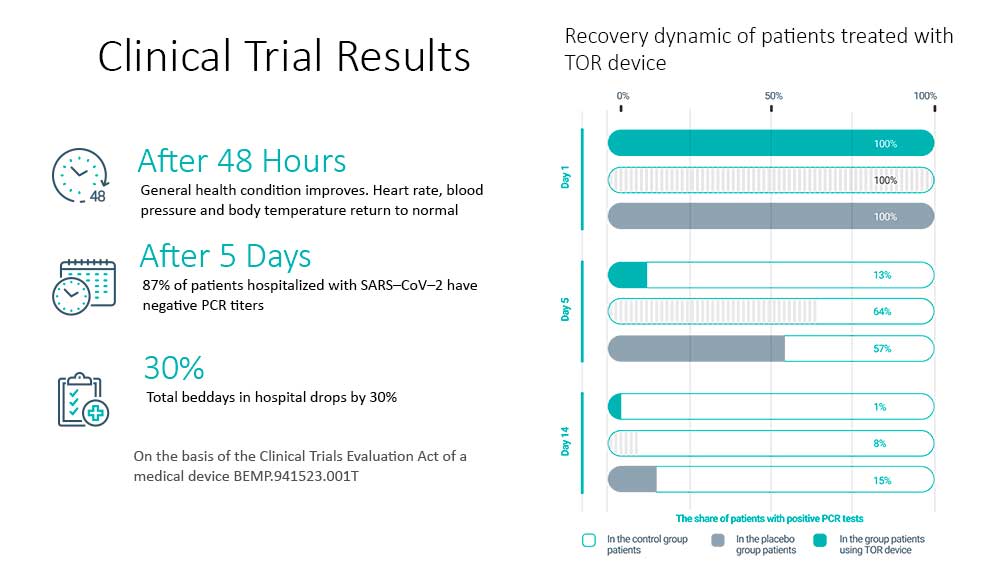
As a result of the study, it was found that “the TOR non-invasive electromagnetic therapy device is safe when used as intended; it is effective as an immune response enhancer in combination with standard COVID-19 treatments to accelerate the elimination of the SARS-CoV-2 virus from the nasopharynx.
Due to the successful completion of clinical trials, the GRANIT Concern, the manufacturer of the TOR device, received the Market Authorization for a medical device No. RZN 2021/15459 issued by Roszdravnadzor on 23.09.2021.
Now the results of clinical trials of the TOR device are in the WHO Global Health Observatory, which makes them available for general use.


 Back
Back


